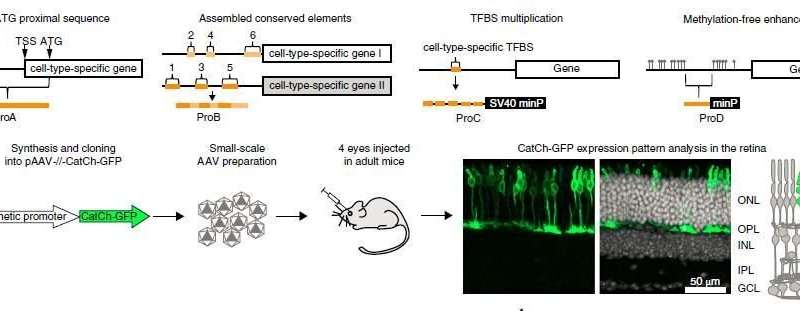
It is a remarkable proof for the concept of IOB: working closely hand in hand, our molecular and clinical researchers have developed a library of 230 adeno associated viral vectors (AAVs), each with a different synthetic promoter. A number of these AAVs specifically target expression to neuronal and glial cell types in the mouse and non-human primate retina in vivo, and in the human retina in vitro.
The library is online, and includes three-dimensional confocal scans. It can be used to search for cell types labeled by active AAVs.
Different potentials for basic and translational research in mice, NHPs and humans
Targeting genes to specific neuronal or glial cell types is valuable both for understanding and repairing brain circuits. AAVs are frequently used for gene delivery, not least because they are safe for the use in human gene therapy and allow efficient and long-lasting transgene expression in target cells. However, targeting expression to specific cell types is an unsolved problem.
“Our resources allow economic, fast and efficient cell-type targeting in a variety of species, both for fundamental science and for gene therapy. Different neuronal and glial cell types of mice, NHPs and humans can be efficiently targeted with our set of AAVs. Subsets are particularly useful in basic research for recording or modulating the activities of cell types, others applicable in translational research for gene therapy of cell type specific human diseases such as retinitis pigmentosa and macular degeneration. We also demonstrate applications for recording and stimulation, as well as for the intersectional and combinatorial labeling of cell types,” says Josephine J眉ttner, first author of the study, and head of the viruses platform in the IOB Molecular Research Center.
While the team could not target chosen cell types, it was able to preferentially target outer or inner retinal cell types with a choice of synthetic promoters. The so-called ProC group of synthetic promoters mostly maintained selectivity across species. It could be particularly useful for translational applications.
Targeting expression in translational research
Cell-type-targeted modulation of brain function and cell-type-specific gene replacement are repair strategies for treating human diseases. For example, in advanced retinal cell degeneration in advanced retinal cell degeneration. Therefore targeting and modulating the remaining retinal circuitry is crucial.
Despite the central importance for both basic and translational research, most current technologies available for cell-type targeting rely on transgenic animals. Either the genetic tool that senses or modulates brain function, or the enzyme which allows the genetic tool to be conditionally expressed, is from the animal’s genome. This limits their applicability. Transgenic components in a cell-type targeting strategy exclude its use in therapy for humans, limit its range of application in preclinical, non-human primate (NHP) research and complicate its use in model organisms such as mice. The development of transgenic NHPs and mice is costly and slow. Moreover, optimizing cell-type targeting in mice yields vectors that are unlikely to optimally target the same cell type in humans.
The new AAV vector library and approach allows testing cell type targeting in human retinas in vitro. The IOB molecular and clinical research teams have shown that this significantly increases the probability that the same vector will target the desired cell type in patients in vivo.
Better understanding of vision and the human brain
Culturing postmortem human brain parts, such as the retina or brain slices, in combination with cell-type targeting AAVs, could lead to a better understanding of the organization and function of cell types in circuits in the human brain. Particularly notable for retinal research is the targeting of photoreceptors with optogenetic tools and other cell types with genetically encoded activity sensors. Although the natural input from light is lost, restoring light sensitivity to photoreceptors may allow computations within the human retina to be studied for several months at the level of cell types and circuits, making the human retina a simple and translationally relevant model system for research.
The study results suggest that the absence of expression in a given cell type or cell class in mice is a useful proxy for the same in humans; therefore, studies in mice can be used to eliminate AAV vectors to be tested in humans.

